An Alternate View of Nuclear Structure
Aran David Stubbs
Contact
Dipole
Dipole is the difference in charge density for 2 halves of the nucleus. By Couloumbs law, energy is lowest where charge is evenly dispersed on the surface of a charged object. In this context, dipole occurs when 1 side of the nucleus is predominantly diquarks, and the other side is predominantly monoquarks. If the monoquarks are all ups, the charge on that side is higher than the charge on the other side. Example: E-1-1L4. This structure has a surface with 50 spheres, 25 monoquarks clumped on 1 side, and 25 diquarks clumped on the other. If all the monoquarks are ups, that side has 50/3 charge, and the other side has 25/3. In order to balance this nucleus, a third of the monoquarks (8) would need to be downs, giving a charge of 26/3 on the monoquark side. This would produce Chlorine 44, but the only stable isotope is Calcium 44 (requiring a total charge of 60/3). Looser structures (with more surface and less interior) support this easily, for instance the symmetric with 8 spheres added. For large nuclei, even a small base dipole can be a problem, since nothing stable over about 175 baryon count has surface downs. In general, the smallest dipole is best, even if a small increase in surface is needed to support it.
Images:
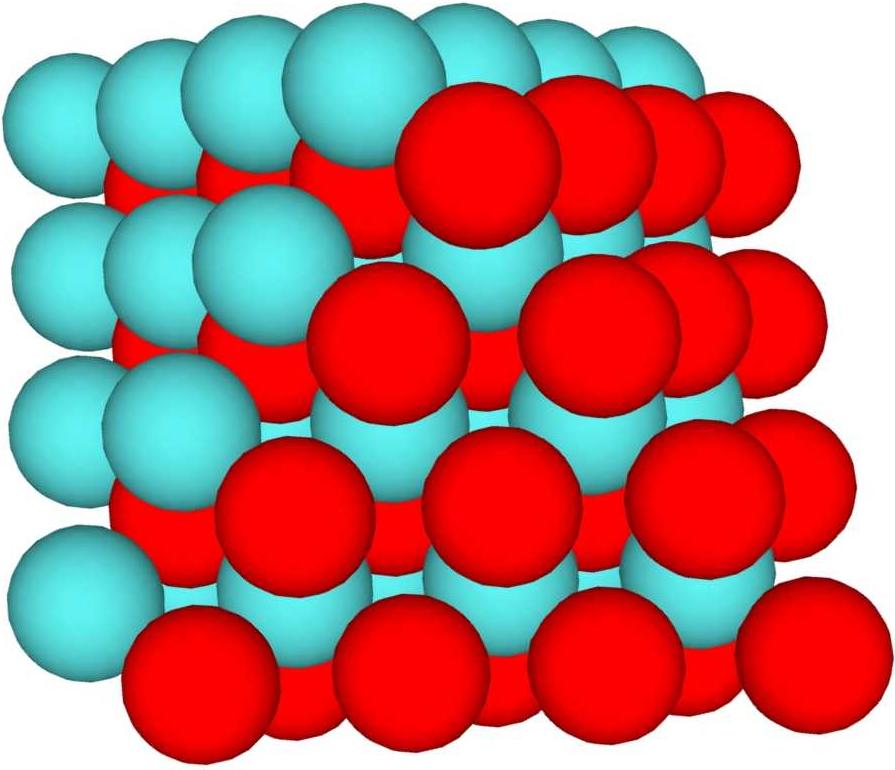 Sphere focused high dipole case: Red monoquarks and blue-green diquarks.
Sphere focused high dipole case: Red monoquarks and blue-green diquarks.
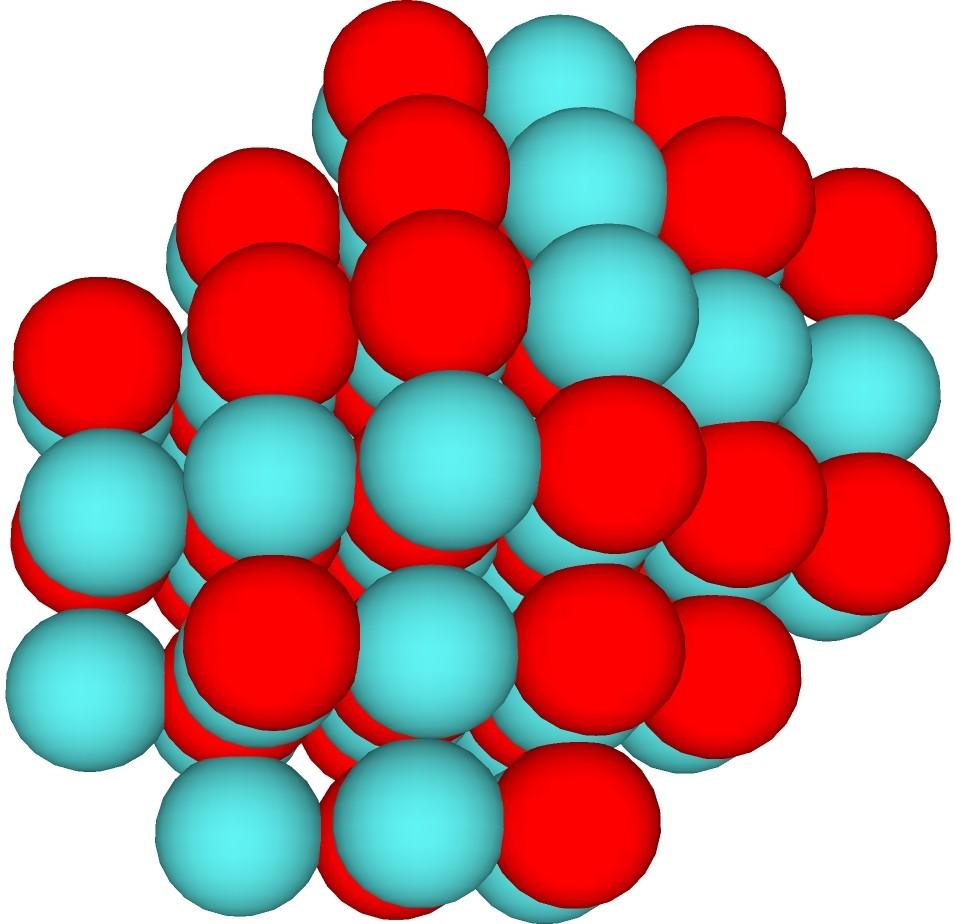 Sphere focused low dipole case: Red monoquarks and blue-green diquarks.
Sphere focused low dipole case: Red monoquarks and blue-green diquarks.


