Brief Comment on How To the Table
of Isotopes in Basic Chemistry and Physics Instruction
Stubbs, D.A. and Clifton, Y.C.
Abstract: This paper presents a compact table of isotopes, which is useful for describing the process of isotope discovery and understanding the energy associated isotopes. Past research has found that the concept of isotopes is difficult to teach. However, it is important because newly discovered isotopes have important applications in medicine and chemistry, so developing useful tools for understanding isotopes has value. The diagram presented here is created specifically to make key concepts related to isotopes comprehensible to students. Previously created tables of isotopes do have utility but are not developed explicitly as aids to instruction.
1. Introduction
It is often difficult for students of basic chemistry to comprehend what an isotope, as opposed to an element or chemical form, means. In one study, where students asked to make sense out the idea of isotopes, the majority of these students were perplexed, and many of the students believed that graphite and diamonds were isotopes of carbon (Schmidt, Baumgärtner, & Eybe, 2003). Another study found that while it is difficult to explain the concept of isotopes, the general process of teaching material involves the teacher transforming his or her content knowledge into something comprehensible by students. (Geddis, Onslow, Beynon & Oesch, 1993). This paper will propose that an efficient way to teach about isotopes is to arrange them in a table, which illustrates many key concepts of isotopes much like the periodic table illustrates key concepts associated with elements. The key concepts this table would teach include, the process of discovering new isotopes and the per baryon energy of an isotope. Unlike many standard tables presenting isotopes, the table presented in this paper does not contain a large number of empty entries. Empty entries could make it difficult for students to find key information. This table is intended, as a means to transform information about isotopes know by scientist, making the information readily comprehensible by students.
Background
History of Isotopes Discovery
It is very valuable for students to understand the process of isotope discovery because of the applications of new isotopes to medicine and chemistry. The on going process of creating new elements has lead to advances in medicine where new radioisotopes (Keevil, 2011). Further, radioactive and non-radioactive isotopes are useful for chemical labeling (Xuan, & Wang, 2008). So, finding a way to illustrate the process of isotope discovery is valuable.
The process of discovering isotopes, which is valuable for students to learn, dates back roughly a century. Prior to 1910, atomic masses were assumed to be constant numbers, and there was no reason to believe that versions of the same atom could have different masses. In 1910, Soddy measured the atomic mass of atoms created by radioactive decay and found that these atoms had a different mass from that found on the periodic table of the elements, thus discovering a distinct isotope for the first time. In 1913, J.J. Thompson was the first to use mass spectroscopy to separate non-radioactive naturally occurring isotopes After these seminal discoveries, there was a great proliferation of discovery of natural isotopes (Budzikiewicz, & Grigsby, 2006). Isotopes can occur in nature if their half-life is long enough to permit the existence of the isotopes since the formation of the solar system or if an isotope is a decay product of another naturally occurring isotope. However, many isotopes that do not occur in nature can be synthesized by fusion or fission processes. The first synthetic isotope was discovered by Irene Joliot Curie and her husband Fredrick Joliot Curie in 1935 (Henricken, & Maillie, 2003) using an early
atomic accelerator. Since that time, there has been a steady process of discovering new synthetic isotopes. In recent years, there has been steady progress in the discovery of new isotopes of transuranic elements (Loveland, 2002). The synthesis of non-naturally occurring elements has had strong applications in medical science (Henricken, & Maillie, 2003). The discovery of various natural and synthetic isotopes has had strong applications in chemistry because the isotopes can be used to label chemical compounds (Hesk, & McNamara, 2007).
Describing the Energy of an Isotope
Energy per baryon is a very useful component way to describe isotopes, because the most common isotopes in the universe is hydrogen which makes up about 75% of the mass of the universe (most of the remaining mass is helium). In the first few minutes of the universe, there was an abundance of hydrogen and neutrons, and some of the neutrons reacted with hydrogen to form deuterium, and the synthesis continued until a significant amount of helium was produced, however, the process was impeded as more neutrons decayed to protons, and as the density of early universe declined freezing the abundance of early isotopes (Olive, Steigman, & Walker, 1999). After the primordial nuclear synthesis a certain amount of elements heavier than hydrogen have been produced notably in the cores of stars and in super novae but the dominant baryonic material of the universe remains hydrogen. Because of the minor amounts of nucleosynthesis since the big bang, the solar hydrogen helium ratio is only slightly more than the primordial ratio and cosmic abundances of other elements remain very low (Vorontsov, Baturin, & Pamiatnykh, 1991).
While there are various ways of presenting the energy of an isotope, the energy per baryon has the advantage of helping the students understand whether the process of creating the isotope in question was an energy releasing process or an energy loosing process. Since the majority of isotopes, with atomic mass greater than 4, were assembled from protons, those which have less energy per baryon than hydrogen, have given off energy during the process of their formation (usually in a star or supernova), and elements with per baryon energy greater than hydrogen have required energy to form.
Existing Tables of Isotopes
The most common form of table describing all atomic isotopes is the plot of number of protons against number of neutrons. This standard table is mostly empty space, making it far bulkier than necessary, and the bulk is productive, and the standard table does little to help students understand the process of discovery of isotopes. This traditional chart is useful to experienced scientists, but was probably not created with instructing students in mind.
The use of the periodic table to illustrate properties of elements was chosen because of the relation of the table to chemical properties. Creating a table of isotopes with similar utility is less straightforward. In the decades following the discovery of isotopes, several publications describing multiple isotopes arranged data in tables. For example Isotopes by Anston 1922), contains several tables, although Anston did not have a single systematic scheme for presenting these isotopes. The common presentation that plots number of neutrons against number of protons goes back to a classic paper by Fea (1935). Fea’s presentation became very common over time, and the endurance of Fea’s scheme for presenting isotope information can be seen on the cover of Firestone’s (1996a, & 1996b) compressive compendium of information about isotopes. Fea (1935) was clearly writing to scientists who already had a strong understanding of the concept of isotopes, and such scientist would not find large amounts of empty space on the able confusing, because in 1935 chemists were still learning to understand isotopes, and the subject was not necessarily taught to beginning students of chemistry. The need to develop a more student centered approach evolved as the applications of isotopes in chemistry, medicine and physics became more important.
The Alternative Table of Isotopes Examination of Possible Arrangement of the Table of Isotopes
We explored using, n, z, A, n-z, n-2z, 2n-3z, and 3n-5z as possible x and y axises for a table of elements. 3 very good choices for tables of elements are given in figures 1, 2 and 3. The colors in all three give the energy per nucleon, placing an emphasis on the energy properties of the isotopes. The data was derived from software from Nucleus-Win Software (2012). Aside from removing empty space from the presentation of isotopes, the figure has the advantage of presenting isotopes in a way such that the most recently discovered isotopes are at the edges and the earlier discovered isotopes are close to the center.
Figure 1 through figure show the graph of A vs. n-2z (which we call the medium arrangement), and figure 4 through figure 6 shows the graph of A vs. n-z (called the long arrangement). Both the long arrangement and the medium arrangement are more compact than the graph of Z vs. n and both of them show distance patterns in terms of energy per baryon.
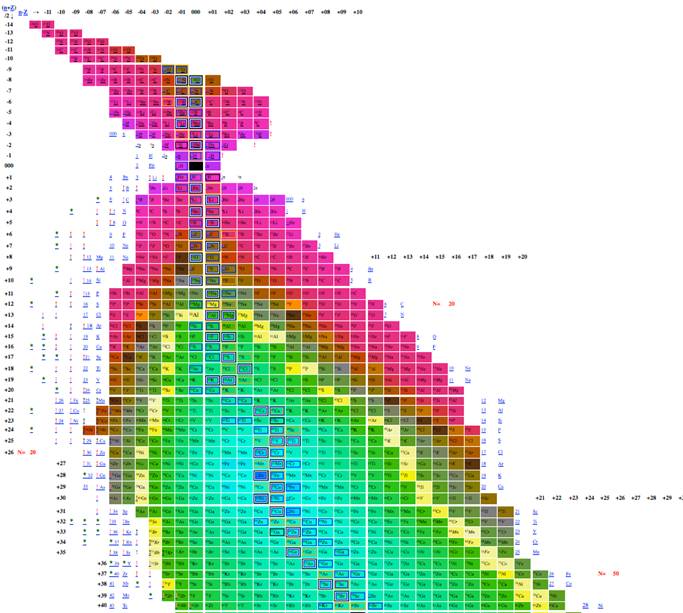
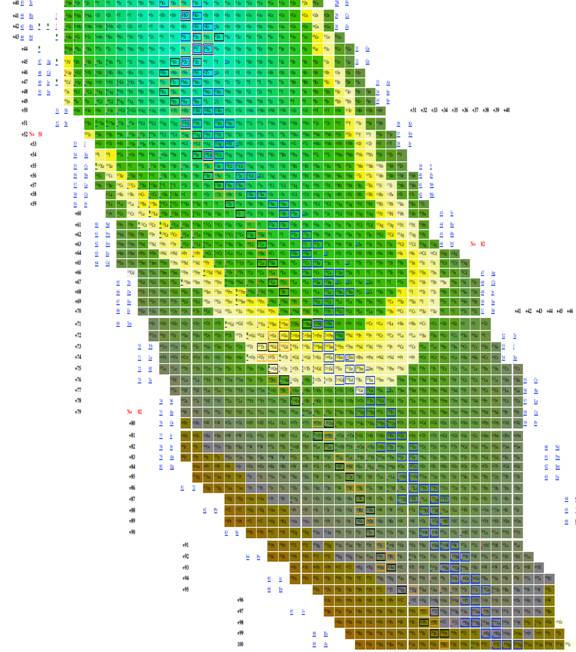
The most effective table was n-z vs. n-2z (figures 7-9) because we found: 1 the arrangement is highly compact; the as will be demonstrated in a later section of this paper the most recently discovered isotopes are at the periphery of the table and the less recently discovered isotopes are towards the center (center defined as close to 56Fe, and 3 the table demonstrates a cyclical structure with respect to energy per isotope. The table also demonstrates a regular periodic structure in terms of the energy per baryon. These relations can help students understand the nature of energy involved in isotope creation and help students understand that the nuclear properties of the isotopes have very different cyclical behavior from the chemical properties of the elements.
The relationship of the Isotopes Table to Discovery of Isotopes
The figure 3 included in this paper presents all known isotopes in a compact form with little empty space. The figure plots n-2z vs. n-z, a mode presentation, which permits the presentation of all known isotopes with very little space. The overall presentation does not mimic that of the periodic table of the elements, but this lack of similarity to periodic table illustrates that nuclear structure (illustrated in tables of isotopes) is very different from electron structure (illustrated in periodic tables).
The data demonstrating that the location on the new table of elements is related to the discovery is shown in the table. The table was divided as follows. The square area surrounding iron 56 was assigned the number one, and the square surrounding the square assigned the number one, was assigned the number 2, and additional numbers were assigned on similar basis. The larger numbered squares contained spaces that were blank, but a large number of elements were present in each square. Each element considered an even numbered distance from the center of the graph was assigned a number. One element at each even numbered distance was selected at random using the excel random number generator. The date that isotopes were discovered was correlated with number associated with their distance. The dates the elements selected were discovered were obtained from Atomic Data and Nuclear Data Tables (Amos, Gross, & Thoennessen, 2011; Amos, & Thoennessen, 2010; Garofali, Robinson, & Thoennessen, 2012; Gross, & Thoennessen, 2012; Kathawa, Fry, & Thoennessen, 2013; May, & Thoennessen, 2012; Nystrom, & Thoennessen, 2012; Shore, Fritsch, Ginepro, Heim, Schuh, & Thoennessen, 2010; Shore, Fritsch, Heim, Schuh, & Thoennessen, 2010; Schuh, Fritsch, Heim, Shore, & Thoennessen, 2010; Thoennessen, 2012a; Thoennessen, 2012b). The correlation between date and distance from 56Fe was r= 0.731. There is no one to one correspondence between date of discovery and distance from 56Fe, but the characteristics that place an isotope farther from 56Fe seem related to what makes discovery take more time, and this can be used to help students understand the process of isotope discovery.
Discussion
This paper presents a useful diagram for teaching about isotopes, but this article also tries to elucidate what information about isotopes is valuable to students, so instructors can work to transform this information into something useful for students. This knowledge can help chemistry teachers formulate lesson plans, and develop their own teaching aids.
References
Amos, S., Gross, J. L., & Thoennessen, M. (2011). Discovery of the calcium, indium, tin, and platinum isotopes. Atomic Data and Nuclear Data Tables, 97(4), 383-402.
Amos, S., & Thoennessen, M. (2010). Discovery of the cadmium isotopes. Atomic Data and Nuclear Data Tables, 96(6), 855-862.
Anston, F.W. (1922). Isotopes, London, UK: Edward Arnold and Company.
Budzikiewicz, H., & Grigsby, R. D. (2006). Mass spectrometry and isotopes: a century of research and discussion. Mass spectrometry reviews, 25(1), 146-157.
CRC Handbook of Chemistry and Physics. 85th ed. CRC Press: Boca Raton, FL.
Fea, G. (1935). Tabelle Riassuntive e Bibliografia Delle Trasmutazioni Artificiali, Nuovo Cimento, 12(6), 368-406.
Firestone (1996a). Table of Isotopes 8th ed. (vo. 1), New York Wiley.
Firestone (1996b). Table of Isotopes 8th ed. (vol. 2), New York Wiley.
Garofali, K., Robinson, R., & Thoennessen, M. (2012). Discovery of chromium, manganese, nickel, and copper isotopes. Atomic Data and Nuclear Data Tables, 98(2), 356-372.
Geddis, A.N., Onslow, B., Benon, C., &Oesch, J. (1993). Transforming content knowledge” Learning to teach about isotopes. Science Education, 77(6), 575-591.
Gross, J. L., & Thoennessen, M. (2012). Discovery of gallium, germanium, lutetium, and hafnium isotopes. Atomic Data and Nuclear Data Tables, 98(5), 983-1002.
Henricken, T., & Maillie, D.H. (2003). Radiation and health. CRC Press: Boca Raton, FL.
Hesk, D., & McNamara, P. (2007). Synthesis of isotopically labeled compounds at Schering‐Plough: an historical perspective. Journal of Labeled
Compounds and Radiopharmaceuticals, 50(9‐10), 875-887.
Kathawa, J., Fry, C., & Thoennessen, M. (2013). Discovery of palladium, antimony, tellurium, iodine, and xenon isotopes. Atomic Data and Nuclear Data Tables, 99(1), 22-52.
Keevil, S. F. (2011). Physics and Medicine 1 Physics and medicine: a historical perspective. Lancet, 379(9825), 1517-24.
May, E., & Thoennessen, M. (2012). Discovery of cesium, lanthanum, praseodymium and promethium isotopes. Atomic Data and Nuclear Data Tables, 98(5), 960-982.
Olive, K.A., Steigman, G. & Walker, T.P. (1999). Primordial nuclear synthesis: Theory and observation. Physics Reports, 333, 389-407. doi:10.1016/S0370-1573(00)00031-4
Nucleus-Win (2012) (Version 2.1) (Software). Paris: CSNSM-Orsay, retrieved from http://nucleus-win.software.informer.com/2.1/
Nystrom, A., & Thoennessen, M. (2012). Discovery of yttrium, zirconium, niobium, technetium, and ruthenium isotopes. Atomic Data and Nuclear Data Tables, 98(2), 95-119.
Schmidt, H. J., Baumgärtner, T., & Eybe, H. (2003). Changing ideas about the periodic table of elements and students’ alternative concepts of isotopes and allotropes. Journal of Research in Science Teaching, 40(3), 257-277.
Shore, A., Fritsch, A., Ginepro, J. Q., Heim, M., Schuh, A., & Thoennessen, M. (2010). Discovery of the barium isotopes. Atomic Data and Nuclear Data Tables, 96(6), 749-758.
Shore, A., Fritsch, A., Heim, M., Schuh, A., & Thoennessen, M. (2010). Discovery of the vanadium isotopes. Atomic Data and Nuclear Data Tables, 96(4), 351-357.
Schuh, A., Fritsch, A., Heim, M., Shore, A., & Thoennessen, M. (2010). Discovery of the iron isotopes. Atomic Data and Nuclear Data Tables, 96(6), 817-823.
Thoennessen, M. (2012 a). Discovery of isotopes with Z≤ 10.” Atomic Data and Nuclear Data Tables 98(1), 43-62.
Thoennessen, M. (2012 b). Discovery of the Isotopes with 11≤ Z≤ 19. Atomic Data and Nuclear Data Tables, 98(5), 933-959.
Vorontsov, S. V., Baturin, V. A., & Pamiatnykh, A. A. (1991). Seismological measurement of solar helium abundance. Nature, 349, 49-51.
Xuan, J. S., & Wang, J. F. (2008). Novel Isotope Labeling Strategies for Protein Solution NMR Spectroscopy: A Review [J]. Chinese Journal of Magnetic Resonance, 3, 020.
|
|
A |
n |
z |
n-z |
n-2z |
2n-3z |
3n-5z |
|
A |
|
A vs. n |
A vs. z |
A vs. n-z |
A vs. n-2z |
A vs. 2n-3z |
A vs. 3n-5z |
|
n |
n vs. A |
|
n vs. z |
n vs. n-z |
n vs. n-2z |
n vs. 2n-3z |
n vs. 3n-5z |
|
z |
z vs. A |
z vs. n |
|
z vs. n-z |
z vs. n-2z |
z vs. 2n-3z |
z vs. 3n-5z |
|
n-z |
n-z vs. A |
n-z vs. n |
n-z vs. z |
|
n-z vs. n-2z |
n-z vs. 2n-3z |
n-z vs. 3n-5z |
|
n-2z |
n-2z vs. A |
n-2z vs. n |
n-2z vs. z |
n-2z vs. n-z |
|
n-2z vs. 2n-3z |
n-2z vs. 3n-5z |
|
2n-3z |
2n-3z vs. A |
2n-3z vs. n |
2n-3z vs. z |
2n-3z vs. n-z |
2n-3z vs. n-2z |
|
2n-3z vs. 3n-5z |
|
3n-5z |
3n-5z vs. A |
3n-5z vs. n |
3n-5z vs. z |
3n-5z vs. n-z |
3n-5z vs. n-2z |
3n-5z vs. 2n-3z |
|
Table 1 Possible Configurations of the table of isotopes.
|
Distance |
Date of Discovery |
|
|
56Fe |
0 |
1923 |
|
62Ni |
2 |
1934 |
|
47V |
4 |
1942 |
|
74Ge |
6 |
1923 |
|
87Y |
8 |
1940 |
|
102Ru |
10 |
1931 |
|
17F |
12 |
1934 |
|
102Pd |
14 |
1935 |
|
130Cs |
16 |
1952 |
|
78Ni |
18 |
1995 |
|
122In |
20 |
1963 |
|
18C |
22 |
1969 |
|
104Sn |
24 |
1985 |
|
118Tc |
26 |
1984 |
|
19B |
28 |
2010 |
|
146Ba |
30 |
1970 |
|
152Cd |
32 |
1990 |
Table II
Randomly selected elements at various distances from 56Fe on the table isotopes, compared to the year the element was discovered. The linear
correlation coefficient is 0.535, implying that there is a strong correlation between the location of the element relative to the center of the table and the
date the element was discovered.
Figure
1. Table of isotopes using revised method. Plotting A/3 vs. n-2z.


Figure
2 Second part of Plotting A/3 vs. n-2z.
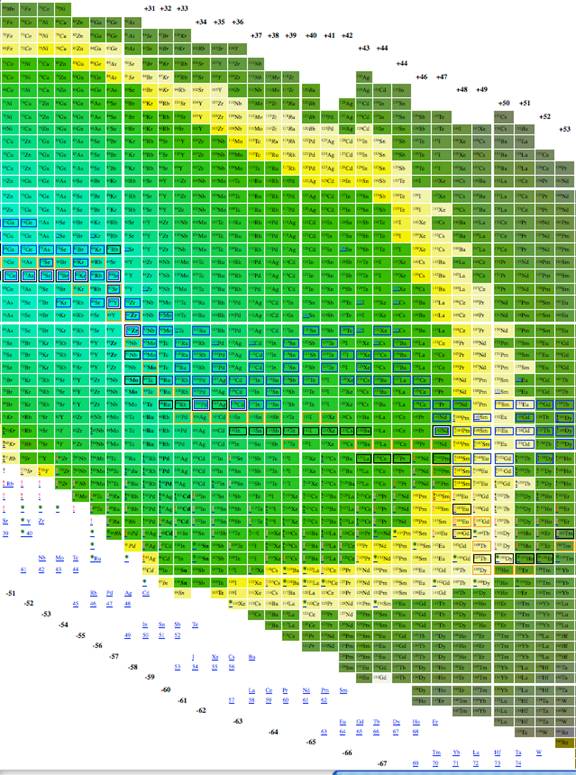
Figure
3. Third part of Plotting A/3 vs. n-2z.
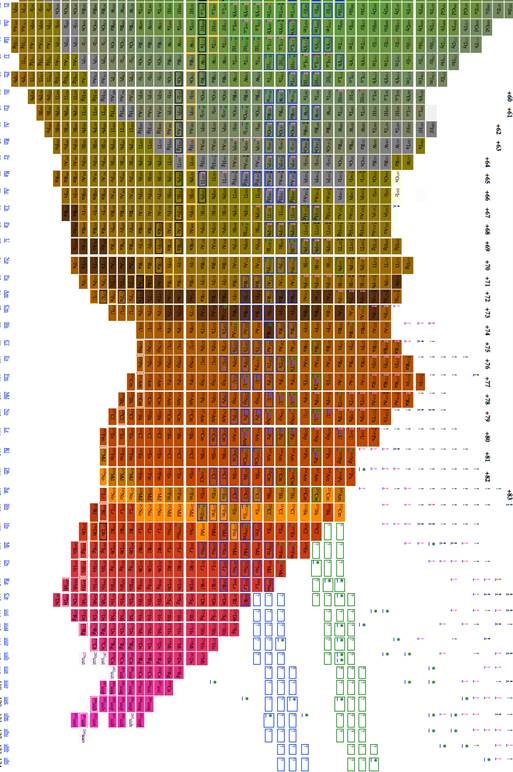
Figure
4 Table of isotopes plotting A/2 vs. n-z

Figure
5 Table of isotopes plotting A/2 vs. n-z
Figure
6. Table of isotopes plotting A/2 vs. n-z
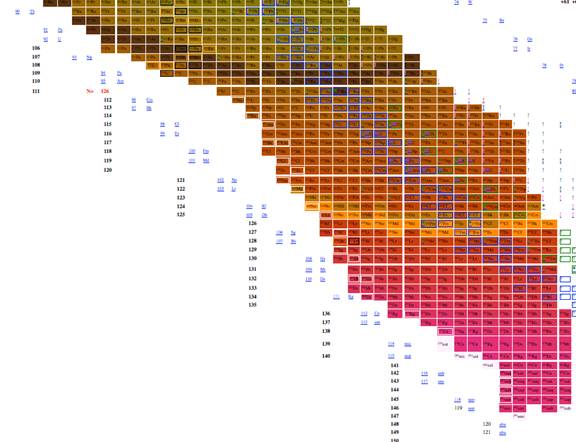
 Figure 7. Table of isotopes
Figure 7. Table of isotopes
plotting n-z vs. n-2z.
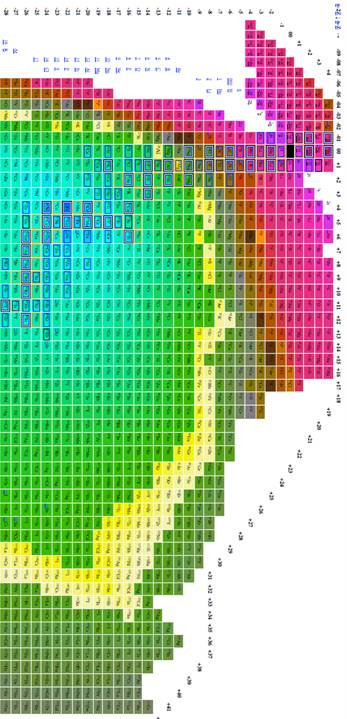
Figure 8
Table of isotopes plotting n-z vs. n-2z.
 |
Figure
9 Table of isotopes plotting n-z vs. n-2z. 


